Frequently asked questions
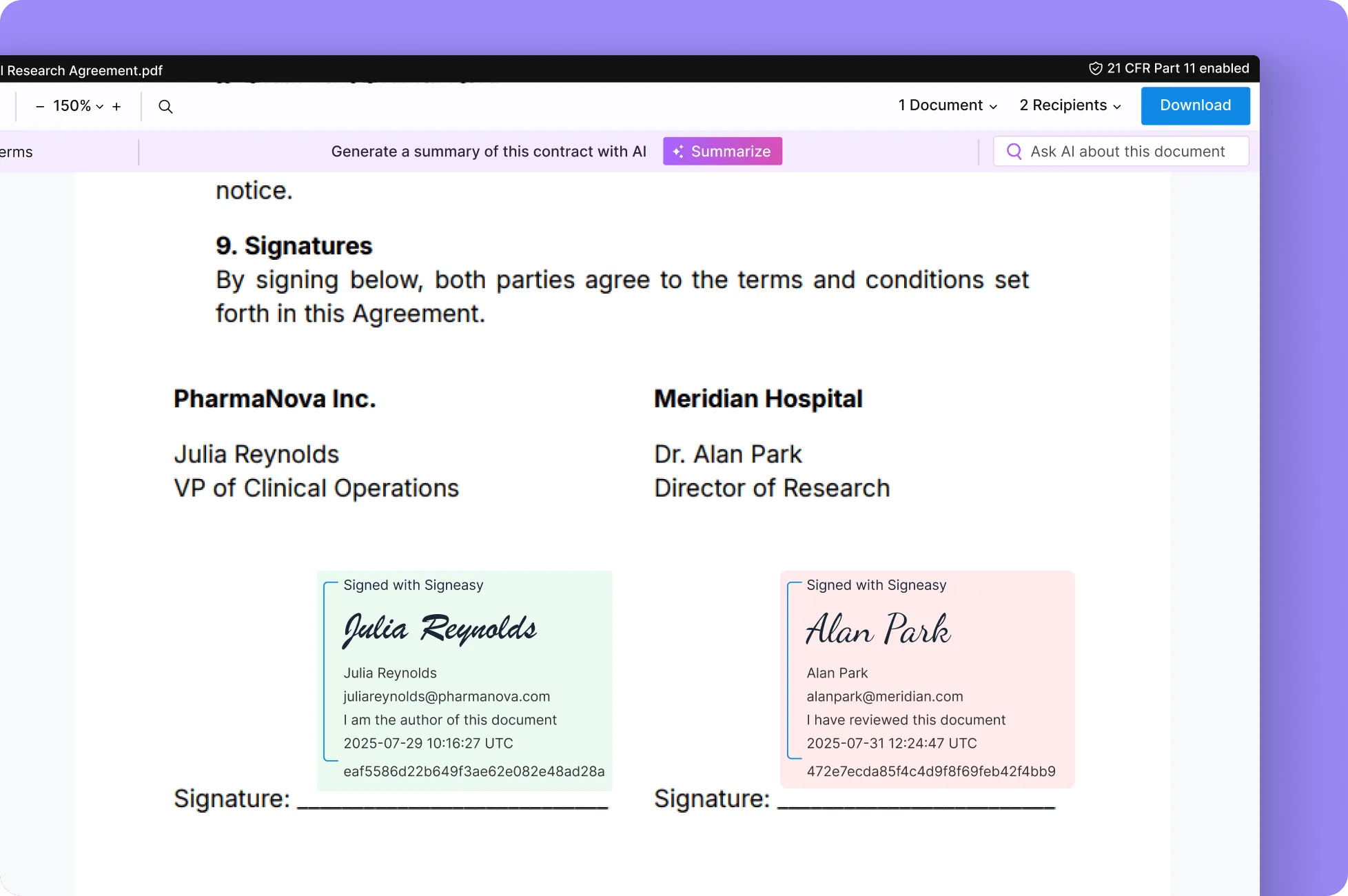
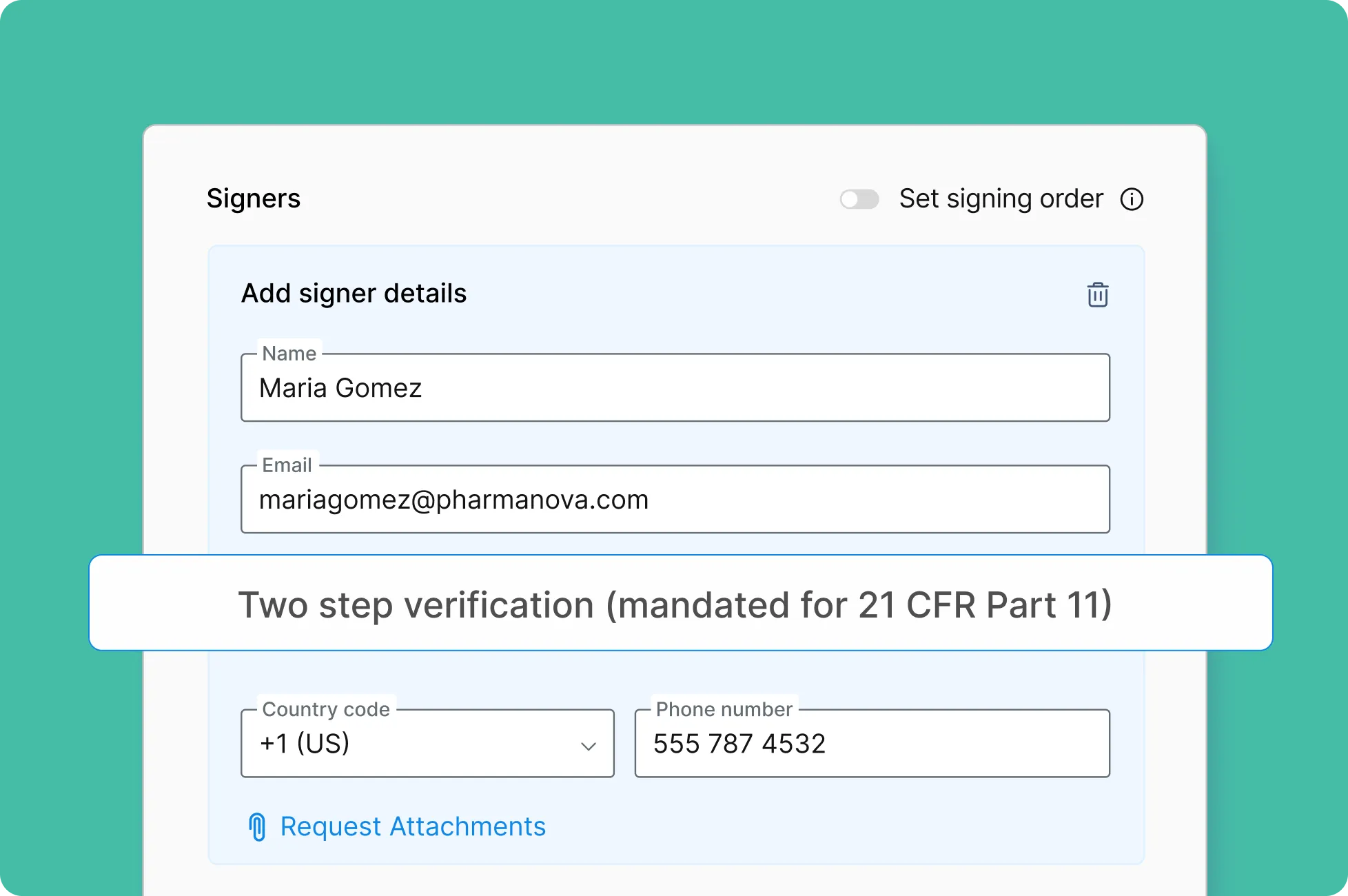
• Signer Authentication — Multifactor authentication before signers access the documents.
• Timestamping — Record when the document was signed and sealed.
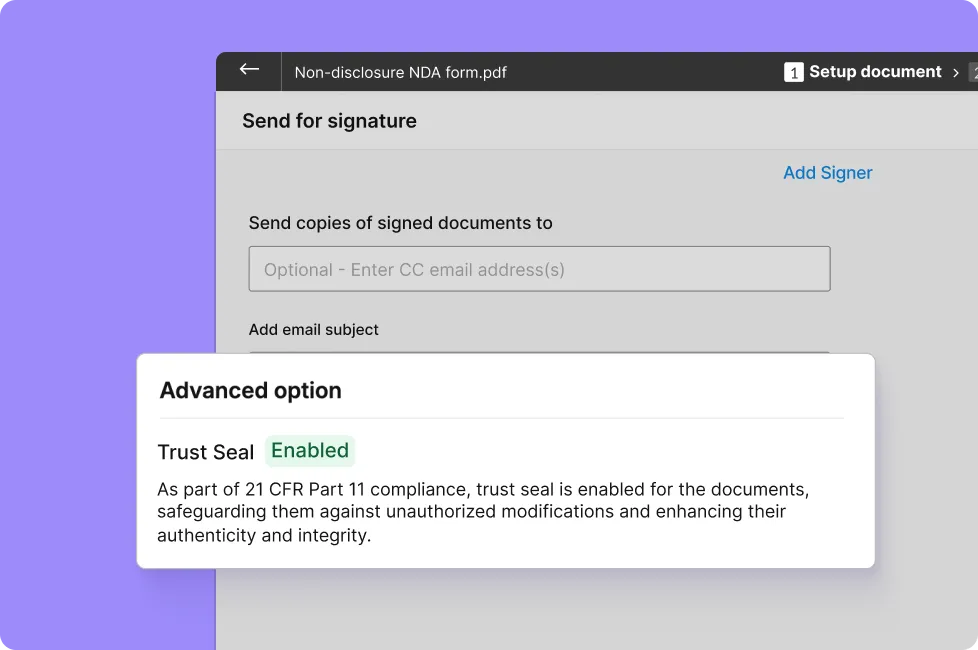
• Tamper-evident PAdES digital seal — Prevent unauthorized edits of the signed documents.
• Signature linking — Signatures are uniquely bound to their respective records.
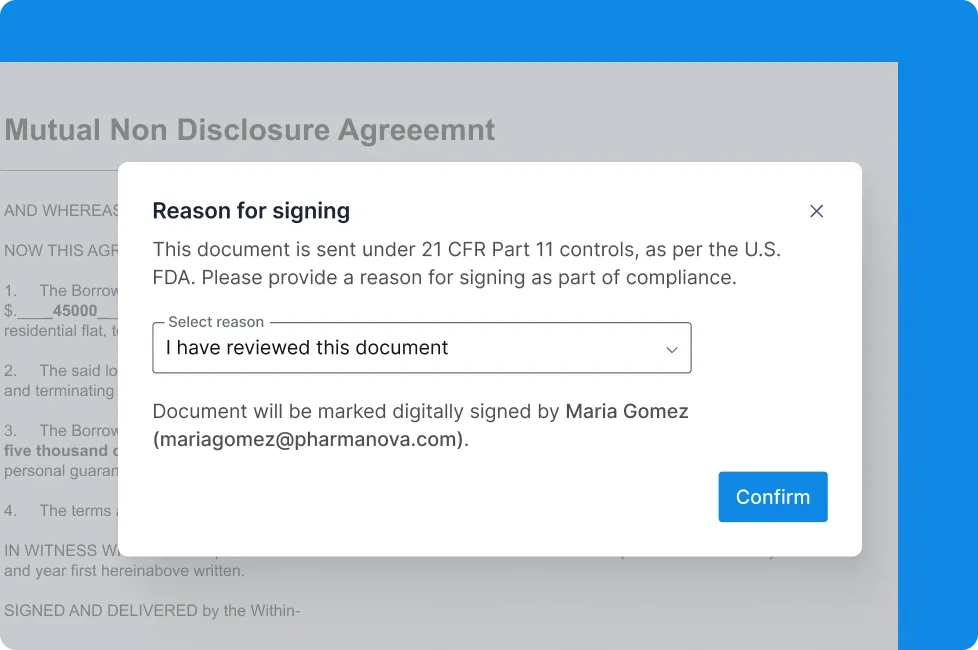
• Signature manifestation — With name, email, and reason for signing.
• Access controls and permissions — Role-based access to protect critical data.
• Comprehensive audit trail — Trail of document events for full traceability of every action.
• Compliant records retention — Store and retrieve compliant documents on demand.
• GLP (Good Laboratory Practice) – covering non-clinical laboratory studies that support research or regulatory submissions.
• GMP (Good Manufacturing Practice) – governing the manufacturing, quality control, and storage of drugs and medical devices.
• GCP (Good Clinical Practice) – ensuring ethical and scientific quality standards for designing, conducting, recording, and reporting clinical trials.
Signeasy provides audit trails, access control, time-stamped signatures, and identity verification features critical for maintaining data integrity and traceability in GxP-compliant workflows.






.webp)




