Today’s regulated businesses are at a crossroads. Many are still tied to paper-based approvals or rely on outdated eSignature tools that weren’t built for FDA compliance. Those who have gone digital often face bloated software that’s hard to use, slows down collaboration, and leaves gaps during audits.
The result? Risky documentation errors, delayed approvals, and mounting pressure to prove compliance, while still trying to move fast and stay efficient.
Signeasy solves for this.
We digitize the entire approval process with built-in 21 CFR Part 11 safeguards that ensure data integrity, reduce manual errors, and facilitate cross-functional, global team collaboration. No more choosing between usability and audit readiness — now, you can have both!
We’re excited to announce that Signeasy now supports 21 CFR Part 11 controls, enabling regulated teams in life sciences, healthcare, and pharmaceuticals to manage documents with the highest standards of security, authenticity, and traceability.
With this launch, your teams can confidently meet the U.S. Food and Drug Administration (FDA) expectations around electronic records and eSignatures without needing complex tools or custom setups.
21 CFR Part 11 in a nutshell
The FDA’s 21 CFR Part 11 regulation ensures that electronic records and signatures are as trustworthy and legally binding as paper documents. It isn’t just a checkbox; it’s table stakes for life sciences companies that embrace digital transformation.
What do you get with Signeasy’s 21 CFR Part 11 controls?
Signeasy’s new 21 CFR Part 11 controls are purpose-built for regulated teams needing agility and compliance. With this launch, here’s what you can expect.
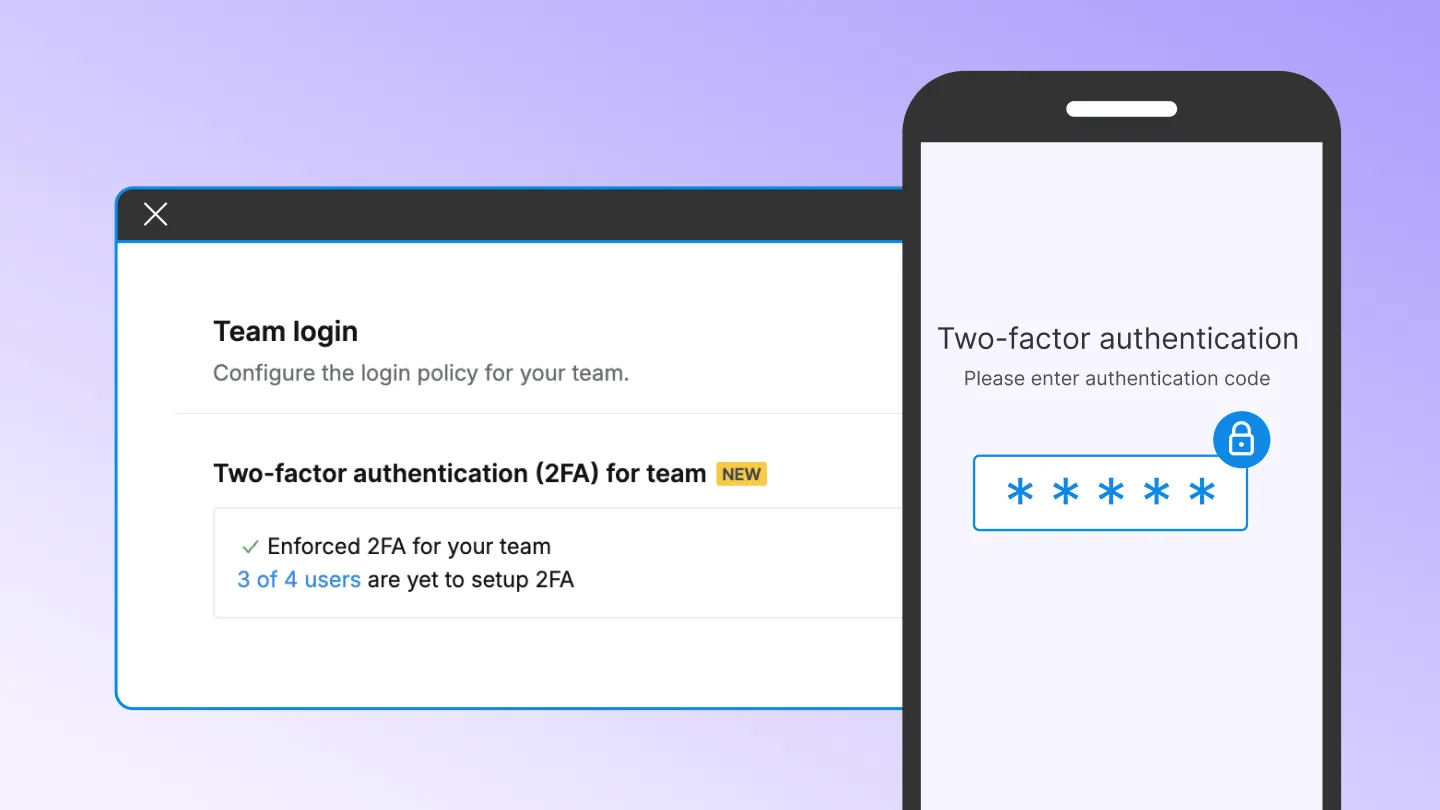
- Signer Authentication: Ensure only verified signers can access documents with Multifactor Authentication (MFA).
- Timestamping: Capture every signature with a timestamp, leaving no room for doubt.
- Tamper-evident digital seals: Protect documents with a PAdES digital seal, making post-signature edits easily detectable.
- Signature linking: Each signature is securely bound to its record, ensuring integrity and traceability.
- Signature manifestation: Clearly display the signer’s name, email, and reason for signing on every document.
- Access controls and permissions: Restrict access to sensitive data with role-based permissions.
- Comprehensive audit trail: Maintain a full log of document events to ensure traceability at every step.
- Compliant records retention: Securely store and retrieve documents whenever you need them.
All of this is delivered through the simple, intuitive experience that Signeasy is known for — without complex setups or cluttered interfaces. You get secure, compliant workflows that scale with your business.
What happens if you don’t comply?
Non-compliance is risky and costly. Your business could face regulatory warning letters, clinical delays, reputational damage, and millions in potential remediation.
If your electronic signature platform can’t meet FDA expectations, every document becomes a liability instead of a workflow accelerator.
Get started with confident compliance
Adopting digital transformation in the life sciences space no longer has to be a compliance headache. With Signeasy’s 21 CFR Part 11-ready controls, you can empower your teams to work efficiently while upholding the highest standards of data integrity and security.
As digital maturity increases across the life sciences industry, so does the burden of proving compliance. Off-the-shelf eSignature tools are no longer enough. Signeasy’s 21 CFR Part 11 support helps your team stay efficient while meeting the highest regulatory standards.
If you’re in a regulated industry and need to stay audit-ready without compromising on usability, Signeasy’s 21 CFR Part 11 controls are now available. Let’s get your workflows compliant and future-proof.